Abstract
Primary Central Nervous System (CNS) involvement of peripheral T-cell lymphoma (PTCL) is a rare phenomenon, with a reported incidence ranging from 2% to 16.7%. Similarly, secondary CNS lymphoma with PTCL phenotype is rare, with an approximately 2-6% incidence. Both clinical situations lead to dismal outcomes. Given the rarity of CNS involvement with PTCL, our understanding of this clinical manifestation is limited to retrospective studies, which have identified risk factors for CNS involvement such as extranodal involvement, stage III-IV disease, bone marrow involvement as well as ATLL subtype. Herein, we characterize CNS involvement of PTCL over a 26-year time period at a single institution, identify if there are any factors that have a positive impact on survival, and extrapolate risk factors for CNS involvement from this dataset.
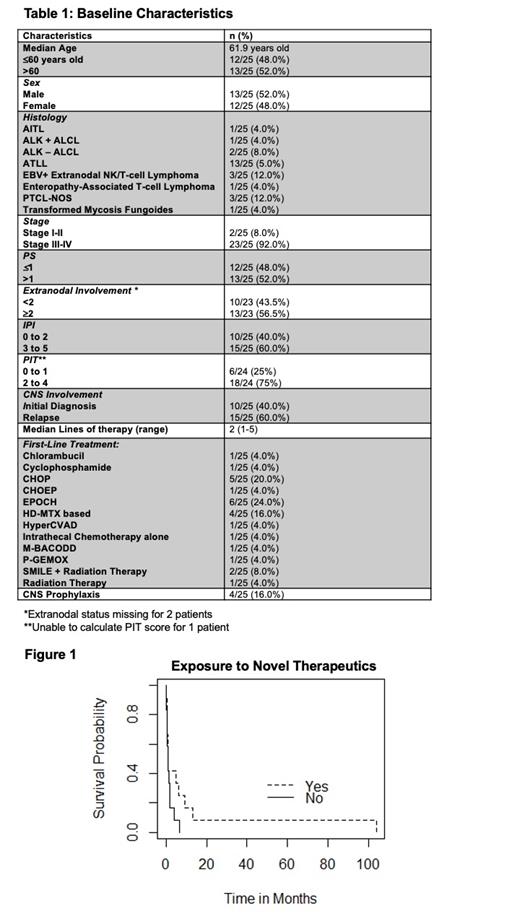
From the years 1996-2020, 222 patients were diagnosed with PTCL, of those, 25 patients developed CNS involvement. All patients had secondary CNS involvement. Baseline characteristics including first-line therapy are noted in Table 1. Four patients received intrathecal methotrexate for CNS prophylaxis, all of which were patients with an ATLL diagnosis. The median time from initial diagnosis to CNS involvement was 4.77 months (0-64 months). Upon CNS diagnosis, the median time to death was 1.13 months. With a median of 2 lines of treatment (range 1-5), 12 patients were exposed to novel therapeutic agents (HDAC inhibitors [HDACis], brentuximab vedotin, pralatrexate) and/or clinical trials investigating epigenetic combinations. Of these patients, 10 patients were exposed to novel therapeutics and/or epigenetic based clinical trials in the 2 nd line setting. There was a trend towards improved overall survival with exposure to novel therapeutics and/or treatment on epigenetic based clinical trials (p=0.1) (Figure 1). With the majority of patients surviving <6 months from CNS diagnosis, there were three excellent responders that survived >12 months, all of which were exposed to novel therapeutics. Two of the three excellent responders were diagnosed with CNS involvement upon initial diagnosis, while 1 patient relapsed in the fourth-line setting with CNS involvement. All three of the excellent responders were exposed to novel therapeutics, particularly pralatrexate as a commonality in the second line setting. Using logistic regression, an ATLL diagnosis, extranodal involvement, and a high ECOG score were associated with a higher risk of CNS involvement. Unlike other studies, a high IPI score was not associated with a predilection for CNS involvement.
In conclusion, we describe one of the largest single-center collection of CNS PTCL involvement. CNS involvement of PTCL is rare, with a reported cumulative incidence in this cohort of 11.3% over a 26-year time period. Risk factors that were identified include an ATLL diagnosis, extranodal involvement, and poor performance status. Exposure to novel therapeutics leads to a trend towards improved outcomes possibly secondary to improved disease control, but overall, the majority of patients had dismal outcomes. Notably, of the novel therapeutics interrogated, romidepsin and pralatrexate have low penetrance into the CNS, while there is no current evidence to suggest brentuximab vedotin is able to cross the blood-brain barrier. The possibility of a disturbed blood-brain barrier to permit improved penetrance of these drugs is plausible, but further studies are warranted to support this. Although a small percentage of patients will suffer from CNS PTCL, there is a dire need to improve therapy for this patient population and to establish universal metrics to identify patients that are at risk of CNS involvement. Moreover, given the rarity of CNS PTCL involvement, a future endeavor would be to validate these findings in larger, multicenter initiative.
Lue: AstraZeneca: Speakers Bureau; Kymera Therapeutics: Research Funding; TG Therapeutics: Consultancy; Epizyme: Consultancy; Kura Oncology: Consultancy. Marchi: Astex: Research Funding; Kyowa Kirin: Honoraria; Merck: Research Funding; BMS: Research Funding; Myeloid Therapeutics: Honoraria; Kymera Therapeutics: Other: Scientific Advisor. O'Connor: Myeloid Therapeutics: Current equity holder in publicly-traded company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Mundipharma: Honoraria; Servier: Research Funding; Kymera: Current equity holder in publicly-traded company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; BMS: Research Funding; Merck: Research Funding; TG Therapeutics: Current Employment, Current equity holder in publicly-traded company.